Search
- Page Path
- HOME > Search
Original Article
- Effect of direct-acting antivirals for hepatitis C virus-related hepatocellular carcinoma recurrence and death after curative treatment
- Young-Hwan Ahn, Heirim Lee, Ji Eun Han, Hyo Jung Cho, Jae Youn Cheong, Bumhee Park, Soon Sun Kim
- J Liver Cancer. 2022;22(2):125-135. Published online June 28, 2022
- DOI: https://doi.org/10.17998/jlc.2022.05.24
- 2,990 Views
- 78 Downloads
- 4 Citations
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Background/Aim
There has been a long-standing debate about the association of directacting antiviral (DAA) therapy and hepatocellular carcinoma (HCC) recurrence. This study aimed to investigate the association between DAA therapy and HCC recurrence after curative therapy.
Methods
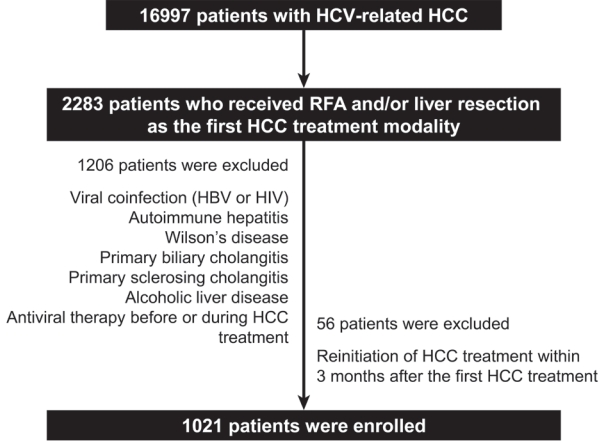
We retrospectively enrolled 1,021 patients with HCV-related (hepatitis C virus) HCC who underwent radiofrequency ablation (RFA), liver resection, or both as the first treatment modality from January 2007 to December 2016 and without a history of HCV therapy before HCC treatment from a nationwide database. The effect of HCV treatment on HCC recurrence and all-cause mortality was also investigated.
Results
Among the 1,021 patients, 77 (7.5%) were treated with DAA, 14 (1.4%) were treated with interferon-based therapy, and 930 (91.1%) did not receive HCV therapy. DAA therapy was an independent prognostic factor for lower HCC recurrence rate (hazard ratio [HR], 0.04; 95% confidence interval [CI], 0.006-0.289; P=0.001 for landmarks at 6 months after HCC treatment and HR, 0.05; 95% CI, 0.007-0.354; P=0.003 for landmarks at 1 year). Furthermore, DAA therapy was associated with lower all-cause mortality (HR, 0.049; 95% CI, 0.007-0.349; P=0.003 for landmarks at 6 months and HR, 0.063; 95% CI, 0.009-0.451; P=0.006 for landmarks at 1 year).
Conclusions
DAA therapy after curative HCC treatment can decrease HCC recurrence and all-cause mortality compared to interferon-based therapy or no antiviral therapy. Therefore, clinicians should consider administering DAA therapy after curative HCC treatment in patients with HCV-related HCC. -
Citations
Citations to this article as recorded by- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma
Jun Il Kim, Jayoun Lee, Gi Hong Choi, Min Woo Lee, Dong Ah Park, Jeong-Ju Yoo
Digestive Diseases and Sciences.2024; 69(3): 1055. CrossRef - Analyzing risk factors and developing a stratification system for hepatocellular carcinoma recurrence after interferon-free direct-acting antiviral therapy in chronic hepatitis C patients
Chih-Hsuan Luan, Pin-Shuo Su, Chi-Jen Chu, Chung-Chi Lin, Chien-Wei Su, Jiing-Chyuan Luo, I-Cheng Lee, Chen-Ta Chi, Shou-Dong Lee, Yuan-Jen Wang, Fa-Yauh Lee, Yi-Hsiang Huang, Ming-Chih Hou
Journal of the Chinese Medical Association.2024; 87(4): 357. CrossRef - Addition of Kidney Dysfunction Type to MELD-Na for the Prediction of Survival in Cirrhotic Patients Awaiting Liver Transplantation in Comparison with MELD 3.0 with Albumin
Kyeong-Min Yeom, Jong-In Chang, Jeong-Ju Yoo, Ji Eun Moon, Dong Hyun Sinn, Young Seok Kim, Sang Gyune Kim
Diagnostics.2023; 14(1): 39. CrossRef - Is direct-acting antiviral treatment beneficial or harmful for patients with hepatitis C virus-related hepatocellular carcinoma?
Hye Won Lee
Journal of Liver Cancer.2022; 22(2): 91. CrossRef
- Comparison of Surgical Resection and Radiofrequency Ablation in Elderly Patients with Hepatocellular Carcinoma

Case Report
- Infiltrative hepatocellular carcinoma with multiple lung metastasis completely cured using nivolumab: a case report
- Ji Eun Han, Hyo Jung Cho, Soon Sun Kim, Jae Youn Cheong
- J Liver Cancer. 2021;21(2):169-176. Published online September 30, 2021
- DOI: https://doi.org/10.17998/jlc.2021.08.26

- 3,141 Views
- 78 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - The current Food and Drug Administration-approved systemic treatments for advanced hepatocellular carcinoma (HCC) include multikinase inhibitors (tyrosine kinase inhibitor [TKI]) and immune checkpoint inhibitors (ICIs). Among ICIs, nivolumab is used as secondline therapy for advanced HCC after sorafenib failure or patient intolerance. In this case, a patient with infiltrative HCC and portal vein tumor thrombosis was treated with hepatic arterial infusion chemotherapy (HAIC) and radiation therapy. New lung metastasis developed after HAICs; thus, lenvatinib treatment was initiated. However, the disease progressed. Thereafter, sorafenib treatment was initiated but he developed intolerance, with grade 3 sorafenib-related diarrhea. Subsequently, nivolumab was administered as rescue therapy. He demonstrated a partial response to nivolumab after the third treatment and viable HCCs in the lungs and liver completely disappeared after the 24th treatment. These findings suggest that nivolumab could be used as an effective rescue therapy for advanced HCC progression after TKI treatment.
-
Citations
Citations to this article as recorded by- Intermediate-stage (BCLC stage B) infiltrative hepatocellular carcinoma: safety and efficacy of chemoembolization
Seong Ho Kim, Jin Hyoung Kim, Gun Ha Kim, Ji Hoon Kim, Heung-Kyu Ko, Hee Ho Chu, Ji Hoon Shin, Dong Il Gwon, Gi-Young Ko, Hyun-Ki Yoon, Shakir Aljerdah, Nayoung Kim
European Radiology.2023; 33(12): 8736. CrossRef
- Intermediate-stage (BCLC stage B) infiltrative hepatocellular carcinoma: safety and efficacy of chemoembolization

Original Article
- Effect of PTEN Polymorphism on the Development of Hepatitis B Virus-associated Hepatocellular Carcinoma
- Soon Sun Kim, Jung Woo Eun, Hyo Jung Cho, Hyun-Young Lee, Chul Won Seo, Gil Ho Lee, So Young Yoon, Choong Kyun Noh, Sung Won Cho, Jae Youn Cheong
- J Liver Cancer. 2019;19(1):46-54. Published online March 31, 2019
- DOI: https://doi.org/10.17998/jlc.19.1.46

- 4,443 Views
- 129 Downloads
- 1 Citation
-
 Abstract
Abstract
 PDF
PDF - Background/Aim
s: Phosphatase and tensin homolog (PTEN) is a known tumor suppressor gene that is downregulated in hepatocellular carcinoma (HCC). Here, we investigated the association between single nucleotide polymorphisms (SNPs) of PTEN and HCC development in patients with hepatitis B virus (HBV) infection.
Methods
Six SNPs of PTEN at positions rs1234221, rs1903860, rs1234220, rs1903858, rs2299941, and rs17431184 were analyzed in a development population (417 chronic HBV carriers without HCC and 281 chronic HBV carriers with HCC). PTEN rs1903858, rs1903860, and rs2299941 SNPs were further assessed for the development of HCC in a validation population of 200 patients with HBV-related liver cirrhosis.
Results
In the development population, PTEN rs1903860 C allele, rs1903858 G allele, and rs2299941 G allele were associated with a low risk of HCC. The haplotype A-T-A-A-A was associated with an increased risk of HCC (recessive model; odds ratio=2.277, 95% confidence interval [CI] =1.144-4.532, P=0.019). In the validation population, PTEN rs2299941 G allele was the only significant protective genetic polymorphism related to HCC development after adjustment for age and sex (hazard ratio=0.582, 95% CI =0.353-0.962, P=0.035).
Conclusions
These findings suggest that genetic polymorphisms in PTEN may affect HCC development in patients with chronic HBV infection. -
Citations
Citations to this article as recorded by- Association of genetic variations in phosphatase and tensin homolog (PTEN) gene with polycystic ovary syndrome in South Indian women: a case control study
Swapna Siddamalla, Suresh Govatati, Veena Kunjumol Venu, Nagendram Erram, Mamata Deenadayal, Sisinthy Shivaji, Manjula Bhanoori
Archives of Gynecology and Obstetrics.2020; 302(4): 1033. CrossRef
- Association of genetic variations in phosphatase and tensin homolog (PTEN) gene with polycystic ovary syndrome in South Indian women: a case control study


 E-submission
E-submission THE KOREAN LIVER CANCER ASSOCIATION
THE KOREAN LIVER CANCER ASSOCIATION

 First
First Prev
Prev



 Follow JLC on Twitter
Follow JLC on Twitter